EMANA Medical
UPNEEQ Eye Drops
UPNEEQ Eye Drops
Couldn't load pickup availability
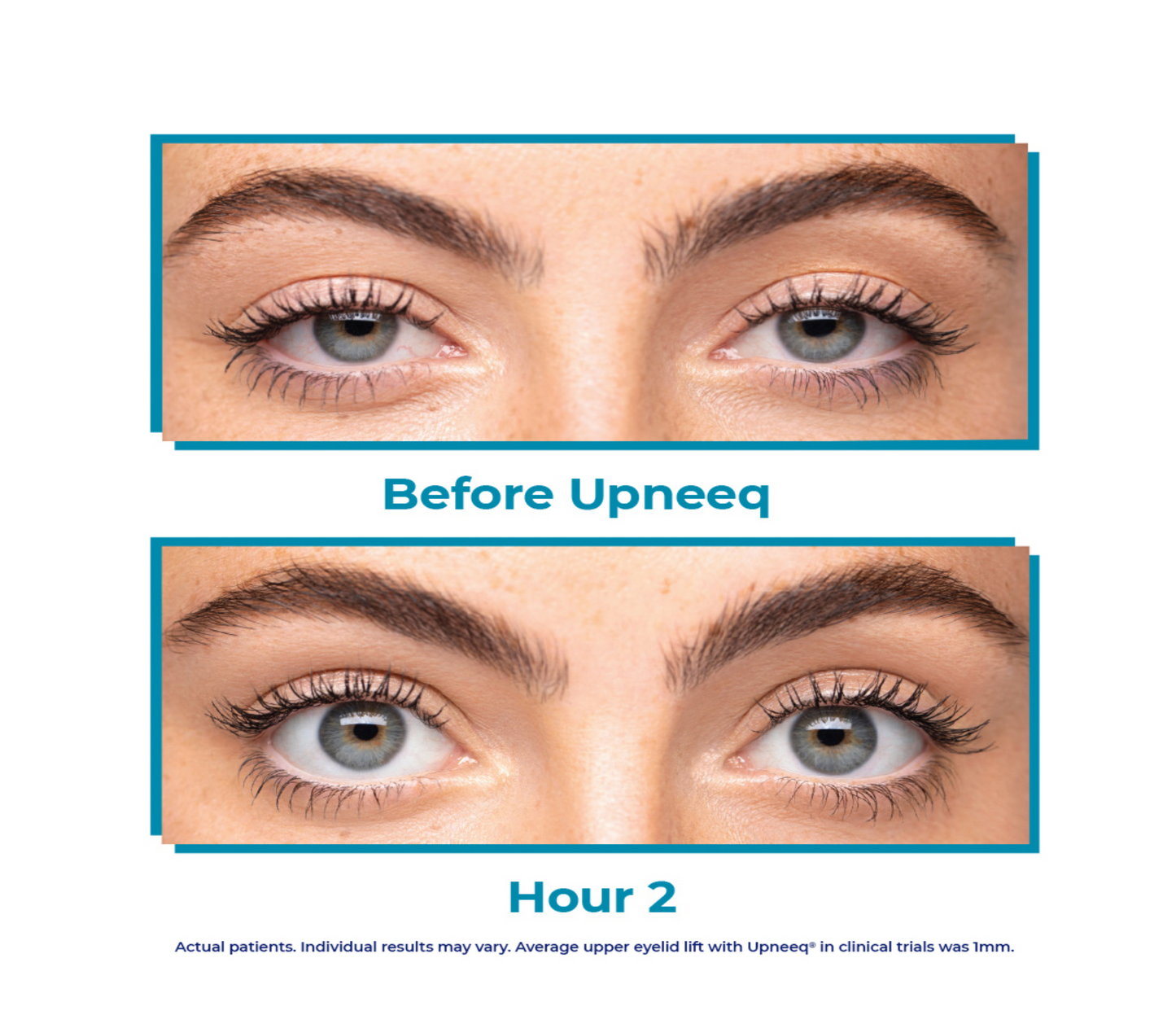
Meet UPNEEQ!
Upneeq is the only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes.
Are low-lying eyelids making your eyes look a little tired? You may have acquired ptosis.
Acquired ptosis (low-lying lids) is a common medical condition that:
- Can develop later in life
- Affects adults of all ages, but occurs more often with increased age
- Usually occurs when the muscles in the eyelid stretch and weaken, causing the upper eyelid to droop
- May be caused by other issues, such as cataract surgery, contact lens wear, or an underlying medical condition. It could also be a sign of a more serious medical condition.
Millions of people who have acquired ptosis may not even be aware of it
- Millions of people over the age of 40 may have acquired ptosis
- But only 15% have been diagnosed, and even fewer have received any treatment*
*Estimate of prevalence was calculated using both U.S. Census data and a published study by Sridharan et al. (1995). The Sridharan study observed a prevalence rate of 11.5% (n=400), which was then applied to the US Census’ projected 2020 population of Americans aged 50+ years.
Can you recognize acquired ptosis (low-lying lids)?

Normal |

Mild |

Moderate |

Severe |
Acquired ptosis can lead to vision impairment
-
Low-lying eyelid(s) can affect eyesight by not only blocking your vision, but also by reducing field of vision, which may interfere with day-to-day functions such as:
- Reading
- Driving
- Computer use
- Looking up without the need to tilt your head back
What is UPNEEQ?
UPNEEQ® (oxymetazoline hydrochloride ophthalmic solution), 0.1% is a prescription eyedrop used to treat acquired blepharoptosis (low-lying lids) in adults.
What warnings and precautions are associated with UPNEEQ?
- Low-lying lids may be related to conditions such as stroke and/or brain aneurysm, Horner syndrome, myasthenia gravis, loss of the ability to move eye muscles, eye infection and eye tumors. Tell your doctor if you have any of these conditions.
- UPNEEQ is a type of medication that may affect your blood pressure. If you have heart disease, uncontrolled high or low blood pressure, or feel faint at rest or when quickly standing up, you should call your doctor if your symptoms get worse.
- Patients with reduced blood flow to the brain or heart, or patients who experience eye or mouth dryness due to an immune system disorder (Sjögren’s syndrome), should use care when taking UPNEEQ. Call your doctor immediately if you feel your symptoms may be getting worse.
- UPNEEQ may increase the risk of eye pressure due to fluid buildup (angle-closure glaucoma) in patients with untreated narrow-angle glaucoma. Call your doctor immediately if you feel increased pressure in your eye after using UPNEEQ.
- Do not let the tip of the UPNEEQ vial touch your eye or any other surface. This can help prevent eye injury or contamination. Each UPNEEQ vial is for one-time use and should be discarded after being used.
Simple, once-daily dosing1
Cut open foil wrapper and remove single-use vial
Apply 1 drop of Upneeq in each affected eye as directed, once a day
- Don’t touch eye with the tip of the vial or touch the tip of the vial to any other surface
- Throw the single-use vial away immediately after applying drops. Vials should not be re-used after opening
Patients who wear contact lenses should remove them before applying Upneeq eyedrops. Contact lenses can be put back in 15 minutes after applying Upneeq.
If more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes between applications.
For complete instructions on using Upneeq, please see the Instructions for Use section in the full Prescribing Information.
Storage requirements: Upneeq should be stored at 68°F-77°F (20°C-25°C) and should be protected from excessive heat.
Keep out of reach of children.
What are the most common side effects of UPNEEQ?
The most common adverse reactions with UPNEEQ (occurring in 1-5% of patients) were eye inflammation, eye redness, dry eye, blurred vision, eye pain at time of use, eye irritation, and headache.
What should my doctor know about before prescribing me UPNEEQ?
- Your doctor should review your full medical history before prescribing UPNEEQ.
- UPNEEQ belongs to a class of medication (alpha-adrenergic agonists) that may affect your blood pressure. Use UPNEEQ carefully if you currently take an alpha-adrenergic agonist medication to treat heart disease or an enlarged prostate. Patients taking beta-blockers, or other medications to treat hypertension or an abnormal heartbeat, should also be careful when using UPNEEQ.
- Patients who use a certain class of antidepressant medication (monoamine oxidase inhibitors) should also be careful when using UPNEEQ, as it may affect the way your body absorbs the medication.
These are not all of the possible side effects of UPNEEQ. Tell your doctor if you have any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects.
To report side effects or product complaints, contact RVL Pharmaceuticals at 1-877-482-3788. You may also report side effects to the FDA by calling 1-800-FDA-1088 or visit www.fda.gov/medwatch.
This is a summary of the most important safety information for UPNEEQ. For more in-depth safety information, please review the full Prescribing Information for UPNEEQ.